The Hormone Aldosterone Promotes The
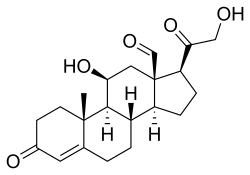 Skeletal formula of the fictitious aldehyde form[1] | |
 Brawl-and-stick model of the 18-acetal-twenty-hemiketal form based on crystallography[ii] [three] | |
| Names | |
|---|---|
| Preferred IUPAC name (1Due south,3aDue south,3bDue south,9aR,9bSouthward,10S,11aR)-ten-Hydroxy-one-(hydroxyacetyl)-9a-methyl-7-oxo-1,two,iii,3a,3b,iv,5,7,8,ix,9a,9b,10,11-tetradecahydro-11aH-cyclopenta[a]phenanthrene-11a-carbaldehyde | |
| Other names Aldocorten; Aldocortin; Electrocortin; Reichstein X; 18-Aldocorticosterone; 18-Oxocorticosterone; 11β,21-Dihydroxy-3,20-dioxopregn-four-en-18-al | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.000.128 |
| IUPHAR/BPS |
|
| KEGG |
|
| MeSH | Aldosterone |
| PubChem CID |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemical formula | C 21 H 28 O 5 |
| Tooth mass | 360.450 k·mol−ane |
| Pharmacology | |
| ATC lawmaking | H02AA01 (WHO) |
| Except where otherwise noted, information are given for materials in their standard country (at 25 °C [77 °F], 100 kPa). Infobox references | |
Aldosterone is the master mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland.[iv] [5] It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon.[6] Information technology plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (Chiliad+) levels. It does so primarily past acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron.[6] Information technology influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retentiveness or loss, blood pressure level, and blood volume.[seven] When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney illness.[8] Aldosterone has exactly the opposite role of the atrial natriuretic hormone secreted by the heart.[7]
Aldosterone is part of the renin–angiotensin–aldosterone arrangement. It has a plasma half-life of less than twenty minutes.[9] Drugs that interfere with the secretion or action of aldosterone are in utilise as antihypertensives, similar lisinopril, which lowers claret pressure by blocking the angiotensin-converting enzyme (ACE), leading to lower aldosterone secretion. The cyberspace effect of these drugs is to reduce sodium and h2o retention but increment memory of potassium. In other words, these drugs stimulate the excretion of sodium and h2o in urine, while they block the excretion of potassium.
Some other instance is spironolactone, a potassium-sparing diuretic of the steroidal spirolactone group, which interferes with the aldosterone receptor (among others) leading to lower blood pressure past the mechanism described above.
Aldosterone was first isolated past Sylvia Tait (Simpson) and Jim Tait in 1953; in collaboration with Tadeusz Reichstein.[10] [11] [12]
Biosynthesis [edit]
The corticosteroids are synthesized from cholesterol within the zona glomerulosa and zona fasciculata of adrenal cortex. Most steroidogenic reactions are catalysed by enzymes of the cytochrome P450 family. They are located inside the mitochondria and require adrenodoxin as a cofactor (except 21-hydroxylase and 17α-hydroxylase).
Aldosterone and corticosterone share the first part of their biosynthetic pathways. The last parts are mediated either past the aldosterone synthase (for aldosterone) or past the 11β-hydroxylase (for corticosterone). These enzymes are nearly identical (they share 11β-hydroxylation and xviii-hydroxylation functions), merely aldosterone synthase is also able to perform an 18-oxidation. Moreover, aldosterone synthase is constitute within the zona glomerulosa at the outer edge of the adrenal cortex; 11β-hydroxylase is found in the zona glomerulosa and zona fasciculata.
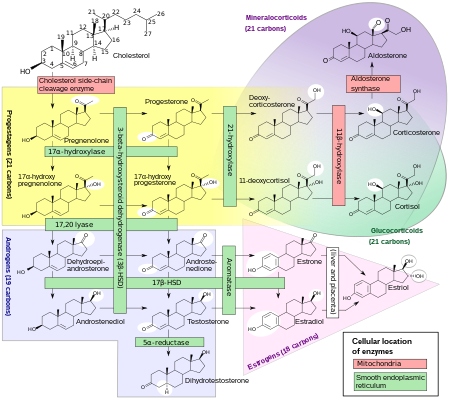
Aldosterone synthase is absent in other sections of the adrenal gland.[ citation needed ]
Stimulation [edit]
Aldosterone synthesis is stimulated past several factors:
- increase in the plasma concentration of angiotensin Three, a metabolite of angiotensin 2
- increment in plasma angiotensin 2, ACTH, or potassium levels, which are present in proportion to plasma sodium deficiencies. (The increased potassium level works to regulate aldosterone synthesis by depolarizing the cells in the zona glomerulosa, which opens the voltage-dependent calcium channels.) The level of angiotensin II is regulated past angiotensin I, which is in turn regulated by renin, a hormone secreted in the kidneys.
- Serum potassium concentrations are the almost strong stimulator of aldosterone secretion.
- the ACTH stimulation test, which is sometimes used to stimulate the production of aldosterone along with cortisol to determine whether main or secondary adrenal insufficiency is present. However, ACTH has just a small role in regulating aldosterone production; with hypopituitarism at that place is no cloudburst of the zona glomerulosa.
- plasma acidosis
- the stretch receptors located in the atria of the middle. If decreased blood pressure is detected, the adrenal gland is stimulated past these stretch receptors to release aldosterone, which increases sodium reabsorption from the urine, sweat, and the gut. This causes increased osmolarity in the extracellular fluid, which volition somewhen return blood pressure toward normal.
- adrenoglomerulotropin, a lipid factor, obtained from pineal extracts. It selectively stimulates secretion of aldosterone.[14]
The secretion of aldosterone has a diurnal rhythm.[xv]
Biological role [edit]
Aldosterone is the main of several endogenous members of the course of mineralocorticoids in humans. Deoxycorticosterone is another of import member of this grade. Aldosterone tends to promote Na+ and water retention, and lower plasma K+ concentration past the following mechanisms:
- Acting on the nuclear mineralocorticoid receptors (MR) within the principal cells of the distal tubule and the collecting duct of the kidney nephron, it upregulates and activates the basolateral Na+/K+ pumps, which pumps three sodium ions out of the cell, into the interstitial fluid and two potassium ions into the cell from the interstitial fluid. This creates a concentration slope which results in reabsorption of sodium (Na+) ions and h2o (which follows sodium) into the claret, and secreting potassium (K+) ions into the urine (lumen of collecting duct).
- Aldosterone upregulates epithelial sodium channels (ENaCs) in the collecting duct and the colon, increasing apical membrane permeability for Na+ and thus absorption.
- Cl− is reabsorbed in conjunction with sodium cations to maintain the arrangement's electrochemical residuum.
- Aldosterone stimulates the secretion of K+ into the tubular lumen.[16]
- Aldosterone stimulates Na+ and water reabsorption from the gut, salivary and sweat glands in substitution for K+.
- Aldosterone stimulates secretion of H+ via the H+/ATPase in the intercalated cells of the cortical collecting tubules
- Aldosterone upregulates expression of NCC in the distal convoluted tubule chronically and its action acutely.[17]
Aldosterone is responsible for the reabsorption of most 2% of filtered sodium in the kidneys, which is nearly equal to the entire sodium content in man blood under normal glomerular filtration rates.[eighteen]
Aldosterone, probably acting through mineralocorticoid receptors, may positively influence neurogenesis in the dentate gyrus.[19]
Mineralocorticoid receptors [edit]
Steroid receptors are intracellular. The aldosterone mineralocorticoid receptor (MR) circuitous binds on the Deoxyribonucleic acid to specific hormone response element, which leads to gene specific transcription. Some of the transcribed genes are crucial for transepithelial sodium transport, including the three subunits of the epithelial sodium channel (ENaC), the Na+/K+ pumps and their regulatory proteins serum and glucocorticoid-induced kinase, and aqueduct-inducing factor, respectively.
The MR is stimulated by both aldosterone and cortisol, but a mechanism protects the body from excess aldosterone receptor stimulation by glucocorticoids (such as cortisol), which happen to be present at much higher concentrations than mineralocorticoids in the healthy private. The mechanism consists of an enzyme chosen xi β-hydroxysteroid dehydrogenase (11β-HSD). This enzyme co-localizes with intracellular adrenal steroid receptors and converts cortisol into cortisone, a relatively inactive metabolite with little affinity for the MR. Liquorice, which contains glycyrrhetinic acrid, can inhibit 11β-HSD and lead to a mineralocorticoid excess syndrome.
Command of aldosterone release from the adrenal cortex [edit]

Major regulators [edit]
The part of the renin–angiotensin system [edit]
Angiotensin is involved in regulating aldosterone and is the core regulation.[21] Angiotensin Two acts synergistically with potassium, and the potassium feedback is near inoperative when no angiotensin II is present.[22] A small-scale portion of the regulation resulting from angiotensin Ii must take identify indirectly from decreased blood catamenia through the liver due to constriction of capillaries.[23] When the claret flow decreases and then does the destruction of aldosterone by liver enzymes.
Although sustained production of aldosterone requires persistent calcium entry through low-voltage-activated Caii+ channels, isolated zona glomerulosa cells are considered nonexcitable, with recorded membrane voltages that are besides hyperpolarized to permit Ca2+ channels entry.[24] Withal, mouse zona glomerulosa cells within adrenal slices spontaneously generate membrane potential oscillations of depression periodicity; this innate electrical excitability of zona glomerulosa cells provides a platform for the product of a recurrent Ca2+ channels signal that tin be controlled by angiotensin Two and extracellular potassium, the 2 major regulators of aldosterone production.[24] Voltage-gated Ca2+ channels have been detected in the zona glomerulosa of the human adrenal, which suggests that Ca2+ channel blockers may directly influence the adrenocortical biosynthesis of aldosterone in vivo.[25]
The plasma concentration of potassium [edit]
The corporeality of plasma renin secreted is an indirect function of the serum potassium[26] [27] as probably adamant past sensors in the carotid avenue.[28] [29]
Adrenocorticotropic hormone [edit]
Adrenocorticotropic hormone (ACTH), a pituitary peptide, also has some stimulating effect on aldosterone, probably by stimulating the formation of deoxycorticosterone, a forerunner of aldosterone.[xxx] Aldosterone is increased past blood loss,[31] pregnancy,[32] and possibly by further circumstances such as physical exertion, endotoxin shock, and burns.[33] [34]
Miscellaneous regulators [edit]
The role of sympathetic nerves [edit]
The aldosterone production is also afflicted to one extent or another by nervous control, which integrates the inverse of carotid artery pressure,[28] hurting, posture,[32] and probably emotion (anxiety, fright, and hostility)[35] (including surgical stress).[36] Anxiety increases aldosterone,[35] which must have evolved because of the time filibuster involved in migration of aldosterone into the prison cell nucleus.[37] Thus, there is an advantage to an creature's anticipating a hereafter demand from interaction with a predator, since too loftier a serum content of potassium has very adverse effects on nervous transmission.
The role of baroreceptors [edit]
Pressure-sensitive baroreceptors are constitute in the vessel walls of nearly all large arteries in the thorax and neck, just are particularly plentiful in the sinuses of the carotid arteries and in the curvation of the aorta. These specialized receptors are sensitive to changes in mean arterial pressure. An increment in sensed force per unit area results in an increased charge per unit of firing by the baroreceptors and a negative feedback response, lowering systemic arterial pressure level. Aldosterone release causes sodium and water retention, which causes increased claret volume, and a subsequent increment in blood pressure, which is sensed by the baroreceptors.[38] To maintain normal homeostasis these receptors also detect low blood pressure or low blood volume, causing aldosterone to be released. This results in sodium retention in the kidney, leading to h2o retentivity and increased claret volume.[39]
The plasma concentration of sodium [edit]
Aldosterone levels vary as an inverse role of sodium intake as sensed via osmotic pressure.[40] The gradient of the response of aldosterone to serum potassium is almost independent of sodium intake.[41] Aldosterone is increased at low sodium intakes, but the charge per unit of increase of plasma aldosterone every bit potassium rises in the serum is not much lower at loftier sodium intakes than it is at depression. Thus, potassium is strongly regulated at all sodium intakes by aldosterone when the supply of potassium is adequate, which it usually is in "archaic" diets.
Aldosterone feedback [edit]
Feedback past aldosterone concentration itself is of a nonmorphological character (that is, other than changes in the cells' number or structure) and is poor, so the electrolyte feedbacks predominate, short term.[33]
Associated clinical weather condition [edit]
Hyperaldosteronism is abnormally increased levels of aldosterone, while hypoaldosteronism is abnormally decreased levels of aldosterone.
A measurement of aldosterone in blood may be termed a plasma aldosterone concentration (PAC), which may be compared to plasma renin activeness (PRA) as an aldosterone-to-renin ratio.
Hyperaldosteronism [edit]
Principal aldosteronism, likewise known as primary hyperaldosteronism, is characterized by the overproduction of aldosterone past the adrenal glands,[42] when not a outcome of excessive renin secretion. Information technology leads to arterial hypertension (loftier blood pressure) associated with hypokalemia, usually a diagnostic clue. Secondary hyperaldosteronism, on the other hand, is due to overactivity of the renin–angiotensin organisation.
Conn'southward syndrome is primary hyperaldosteronism caused by an aldosterone-producing adenoma.
Depending on crusade and other factors, hyperaldosteronism can be treated past surgery and/or medically, such as by aldosterone antagonists.
The ratio of renin to aldosterone is an effective screening test to screen for master hyperaldosteronism related to adrenal adenomas.[43] [44] It is the near sensitive serum blood test to differentiate principal from secondary causes of hyperaldosteronism.[45] Blood obtained when the patient has been standing for more than 2 hours are more sensitive than those from when the patient is lying down. Before the test, individuals should not restrict salt and low potassium should be corrected before the test considering it tin can suppress aldosterone secretion.[45]
Hypoaldosteronism [edit]
An ACTH stimulation examination for aldosterone tin aid in determining the cause of hypoaldosteronism, with a low aldosterone response indicating a primary hypoaldosteronism of the adrenals, while a large response indicating a secondary hypoaldosteronism. The nigh common cause of this condition (and related symptoms) is Addison's affliction; it is typically treated by fludrocortisone, which has a much longer persistence (ane day) in the bloodstream.
Additional images [edit]
-

Corticosteroid biosynthetic pathway in rat
-
References [edit]
- ^ Singh, Neeraj; Taibon, Judith; Pongratz, Stephan; Geletneky, Christian (2021). "Accented content determination by quantitative NMR (qNMR) spectroscopy: a curious case of aldosterone". RSC Adv. eleven (38): 23627–23630. Bibcode:2021RSCAd..1123627S. doi:10.1039/D1RA03472C. PMC9036601. PMID 35479823.
- ^ "CSD Entry: ALDAHA10". Cambridge Structural Database: Admission Structures. Cambridge Crystallographic Information Centre. 1972. Retrieved 2022-09-03 .
- ^ Duax, William L.; Hauptman, Herbert (1972). "Crystal structure and molecular conformation of aldosterone". J. Am. Chem. Soc. 94 (15): 5467–5471. doi:ten.1021/ja00770a050. PMID 5040851.
- ^ Jaisser F, Farman N (January 2016). "Emerging Roles of the Mineralocorticoid Receptor in Pathology". Pharmacological Reviews. 68 (i): 49–75. doi:10.1124/pr.115.011106. PMID 26668301.
- ^ Marieb, Elaine Nicpon; Hoehn, Katja (2013). "Affiliate 16". Man anatomy & physiology (9th ed.). Boston: Pearson. pp. 629, Question fourteen. OCLC 777127809.
- ^ a b Arai, Keiko; Chrousos, George P. (2000-01-01). "Aldosterone Deficiency and Resistance". In De Groot, Leslie J.; Chrousos, George; Dungan, Kathleen; Feingold, Kenneth R.; Grossman, Ashley; Hershman, Jerome M.; Koch, Christian; Korbonits, Márta; McLachlan, Robert (eds.). Endotext. S Dartmouth (MA): MDText.com, Inc. PMID 25905305.
- ^ a b Marieb Human Anatomy & Physiology 9th edition, affiliate:sixteen, page:629, question number:fourteen
- ^ Gajjala, Prathibha Reddy; Sanati, Maryam; Jankowski, Joachim (2015-07-08). "Cellular and Molecular Mechanisms of Chronic Kidney Disease with Diabetes Mellitus and Cardiovascular Diseases every bit Its Comorbidities". Frontiers in Immunology. 6: 340. doi:10.3389/fimmu.2015.00340. ISSN 1664-3224. PMC4495338. PMID 26217336.
- ^ "Pharmacokinetics of Corticosteroids". 2003. Retrieved 15 June 2016.
- ^ Connell, John Thou. C.; Davies, Eleanor (2005-07-01). "The new biology of aldosterone". Journal of Endocrinology. 186 (one): 1–20. doi:x.1677/joe.one.06017. ISSN 0022-0795. PMID 16002531.
- ^ Tait, Sylvia A.S; Tait, James F; Coghlan, John P (2004-03-31). "The discovery, isolation and identification of aldosterone: reflections on emerging regulation and part". Molecular and Cellular Endocrinology. 217 (1–2): ane–21. doi:10.1016/j.mce.2003.10.004. PMID 15134795. S2CID 5738857.
- ^ Williams JS, Williams GH (June 2003). "50th anniversary of aldosterone". J Clin Endocrinol Metab. 88 (6): 2364–72. doi:10.1210/jc.2003-030490. PMID 12788829.
- ^ Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (ane). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- ^ Farrell G (May 1960). "Adrenoglomerulotropin". Circulation. 21 (5): 1009–15. doi:10.1161/01.CIR.21.5.1009. PMID 13821632.
- ^ Hurwitz S, Cohen RJ, Williams GH (April 2004). "Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest". J Appl Physiol. 96 (4): 1406–14. doi:10.1152/japplphysiol.00611.2003. PMID 14660513.
- ^ Palmer, LG; Frindt, Grand (2000). "Aldosterone and potassium secretion past the cortical collecting duct". Kidney International. 57 (iv): 1324–eight. doi:x.1046/j.1523-1755.2000.00970.x. PMID 10760062.
- ^ Ko, Benjamin; Mistry, Abinash C.; Hanson, Lauren; Mallick, Rickta; Wynne, Brandi Chiliad.; Thai, Tiffany L.; Bailey, James L.; Klein, Janet D.; Hoover, Robert Southward. (2013-09-01). "Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway". American Journal of Physiology. Renal Physiology. 305 (5): F645-652. doi:10.1152/ajprenal.00053.2013. ISSN 1522-1466. PMC3761211. PMID 23739593.
- ^ Sherwood, Lauralee (2001). Homo physiology: from cells to systems. Pacific Grove, CA: Brooks/Cole. ISBN0-534-56826-2. OCLC 43702042.
- ^ Fischer AK, von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czéh B (Baronial 2002). "The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat". Brain Res. 947 (two): 290–3. doi:10.1016/S0006-8993(02)03042-1. PMID 12176172. S2CID 24099239.
- ^ Page 866-867 (Integration of Table salt and H2o Residue) and 1059 (The Adrenal Gland) in: Walter F. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBNone-4160-2328-3.
- ^ Williams GH, Dluhy RG (November 1972). "Aldosterone biosynthesis. Interrelationship of regulatory factors". Am J Med. 53 (five): 595–605. doi:10.1016/0002-9343(72)90156-8. PMID 4342886.
- ^ Pratt JH (September 1982). "Role of angiotensin II in potassium-mediated stimulation of aldosterone secretion in the domestic dog". J Clin Invest. seventy (3): 667–72. doi:10.1172/JCI110661. PMC370270. PMID 6286729.
- ^ Messerli FH, Nowaczynski West, Honda M, et al. (February 1977). "Effects of angiotensin 2 on steroid metabolism and hepatic blood period in man". Apportionment Research. 40 (2): 204–7. doi:10.1161/01.RES.40.2.204. PMID 844145.
- ^ a b Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ (June 2012). "Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electricaloscillators". J Clin Invest. 122 (6): 2046–2053. doi:10.1172/JCI61996. PMC3966877. PMID 22546854.
- ^ Felizola SJ, Maekawa T, Nakamura Y, Satoh F, Ono Y, Kikuchi One thousand, Aritomi S, Ikeda M, Yoshimura Yard, Tojo K, Sasano H (2014). "Voltage-gated calcium channels in the man adrenal and primary aldosteronism". J Steroid Biochem Mol Biol. 144 (part B): 410–416. doi:10.1016/j.jsbmb.2014.08.012. PMID 25151951. S2CID 23622821.
- ^ Bauer JH, Gauntner WC (March 1979). "Upshot of potassium chloride on plasma renin activity and plasma aldosterone during sodium brake in normal man". Kidney Int. 15 (three): 286–93. doi:10.1038/ki.1979.37. PMID 513492.
- ^ Linas SL, Peterson LN, Anderson RJ, Aisenbrey GA, Simon FR, Berl T (June 1979). "Mechanism of renal potassium conservation in the rat". Kidney Int. 15 (6): 601–eleven. doi:10.1038/ki.1979.79. PMID 222934.
- ^ a b Gann DS Mills IH Bartter 1960 On the hemodynamic parameter mediating increase in aldosterone secretion in the canis familiaris. Fed. Proceedings xix; 605–610.
- ^ Gann DS, Cruz JF, Casper AG, Bartter FC (May 1962). "Mechanism by which potassium increases aldosterone secretion in the dog". Am J Physiol. 202 (5): 991–6. doi:ten.1152/ajplegacy.1962.202.five.991. PMID 13896654.
- ^ Brown RD, Strott CA, Liddle GW (June 1972). "Site of stimulation of aldosterone biosynthesis by angiotensin and potassium". J Clin Invest. 51 (6): 1413–8. doi:10.1172/JCI106937. PMC292278. PMID 4336939.
- ^ Ruch TC Fulton JF 1960 Medical Physiology and Biophysics. W.B. Saunders and Co., Phijl & London. On p1099.
- ^ a b Farrell G (October 1958). "Regulation of aldosterone secretion". Physiological Reviews. 38 (four): 709–28. doi:x.1152/physrev.1958.38.iv.709. PMID 13590935.
- ^ a b Vecsei, Pál; Gláz, Edith (1971). Aldosterone. New York: Pergamon Printing. ISBN0-08-013368-i. OCLC 186705.
- ^ Farrell GL, Rauschkolb EW (November 1956). "Evidence for diencephalic regulation of aldosterone secretion". Endocrinology. 59 (5): 526–31. doi:10.1210/endo-59-v-526. PMID 13375573. on 529
- ^ a b Venning EH, DyrenfurthY I, Brook JC (Baronial 1957). "Consequence of anxiety upon aldosterone excretion in man". J Clin Endocrinol Metab. 17 (8): 1005–eight. doi:10.1210/jcem-17-eight-1005. PMID 13449153.
- ^ Elman R, Shatz BA, Keating RE, Weichselbaum TE (July 1952). "Intracellular and Extracellular Potassium Deficits in Surgical Patients". Register of Surgery. 136 (1): 111–31. doi:10.1097/00000658-195208000-00013. PMC1802239. PMID 14934025.
- ^ Sharp GUG Leafage A 1966 in; Recent Progress in Hormone Research. (Pincus G, ed.
- ^ Copstead, E. C. & Banasik, J. L. (2010.) Pathophysiology. (4th ed.). St. Louis, Mo: Saunders Elsevier.
- ^ Marieb, E. N. (2004) Human anatomy and physiology (6th ed) San Francisco: Pearson Benjamin Cummings.
- ^ Schneider EG, Radke KJ, Ulderich DA, Taylor RE (April 1985). "Effect of osmolality on aldosterone secretion". Endocrinology. 116 (4): 1621–six. doi:x.1210/endo-116-4-1621. PMID 3971930.
- ^ Dluhy RG, Axelrod 50, Underwood RH, Williams GH (August 1972). "Studies of the control of plasma aldosterone concentration in normal man: II. Upshot of dietary potassium and acute potassium infusion". J Clin Invest. 51 (8): 1950–7. doi:10.1172/JCI107001. PMC292351. PMID 5054456.
- ^ Conn JW, Louis LH (1955). "Primary aldosteronism: a new clinical entity". Trans. Assoc. Am. Physicians. 68: 215–31, discussion, 231–3. PMID 13299331.
- ^ Rayner, BL (2000). "The aldosterone/renin ratio as a screening test for primary aldosteronism". Southward Afr Med J. 90 (iv): 394–400. PMID 10957926.
- ^ Ducher, M; Mounier-Véhier, C; Baguet, JP; Tartière, JM; Sosner, P; Régnier-Le Coz, Southward; Perez, L; Fourcade, J; Jabourek, O; Lejeune, S; Stolz, A; Fauvel, JP (December 2012). "Aldosterone-to-renin ratio for diagnosing aldosterone-producing adenoma: a multicentre study". Athenaeum of Cardiovascular Diseases. 105 (12): 623–30. doi:ten.1016/j.acvd.2012.07.006. PMID 23199617.
- ^ a b Hoffman, Robert (Oct 19, 2018). "What is the office of aldosterone-to-renin ratio (ARR) in the diagnosis of hyperaldosteronism?". www.medscape.com . Retrieved 18 May 2019.
The Hormone Aldosterone Promotes The,
Source: https://en.wikipedia.org/wiki/Aldosterone
Posted by: collinbroddy.blogspot.com


0 Response to "The Hormone Aldosterone Promotes The"
Post a Comment